The methods of cracking chain molecules in petroleum have expanded over the past century just as every other invention and process has been modified for the benefit of the consumer. Thermal cracking was developed one hundred years ago as a way to salvage more useful products (gasoline, naphtha, diesel) from the tower residues and heavy fractions. This was the first commercial conversion process. By using hydrogen abstraction and beta scission, engineers were able to “chop up” the long chain alkanes that are the responsible for the heaviness of the residue into shorter C-H chains. Although the first scientists may not have completely known the chemistry of the reaction, they knew that this process was going to be a crucial part of petroleum refineries, especially with the boom of automobiles in the early 20th century. The influx of automobiles may have been responsible for the beginning of the use of thermal cracking, but it also may have been its demise. The process is barely ever used for increasing the yield of lighter products since it proved to produce low octane numbers (compared to its more chemically accurate counterpart, catalytic cracking). Thermal cracking is still used in today’s refineries, yet most of the time it is utilized for the production of ethylene.
Category Archives: Week 06
Thermal Cracking
Thermal cracking was initially developed to attempt to solve an energy source issue for the automobile and for aircrafts. Gasoline is produced by cracking gas oils at high temperature and initially at high pressures for hydrogen abstraction, then at low pressures for the cracking or breaking of the bonds. The heavy and light gas oils are separated to avoid heating the reactive longer alkane chains to maintain coke formations. This was a great means for our country to produce light middle distillates and heavier ends by excessive heating. The US has since discovered catalytic cracking which allows for a conversion process that produces higher yields of gasoline and higher octane number. Although the process of thermal cracking is in the past for the US, other countries still use this conversion process as a principal application of petroleum refining for diesel fuel production.
Visbreaking is known as a mild thermal cracking process which reduces the viscosity of the vacuum distillation residue to produce fuel oil, which is converted to lighter distillates. Thermal severity is a measurement of temperature and time and is an index number that helps describe how viscosity will change in visbreaking. Thermal severity can inform a refinery on how asphaltene and carbon content of feedstocks are characteristics to be aware of when considering possibilities of coking in the visbreaker reactor.
Thermal Cracking Past & Present
Blog 6
Write a post reviewing the significance of thermal cracking in petroleum refining in the past and present.
After all of the various physical separations have occurred to a crude oil, such as distillation, deasphalting, and dewaxing, there is a need to now change the composition of the crude oil using chemistry, breaking and creating bonds. The yields of this product after just undergoing the physical changes does not meet the demand required so further chemical separations must be pursued. The earliest discovered method for chemical separation is known as thermal cracking where the chemical bonds are broken through changing temperature.
In the past the large demand for gasoline as a product from crude oil started with the mass produced model T automobile back in the 1920’s. Thermal cracking was the first chemical process introduced to convert heavier hydrocarbons with longer chain paraffins to lighter distillates. Thermal cracking produces shorter straight chain alkanes from longer straight chains. This was the number one method used to obtain gasoline from crude oil back in the day. It does so by using brutal heat, heating the temperatures until the compounds crack and the chemical bonds are broken. This process then delivered the gasoline, with a low octane rating, needed for automobiles at that time.
Thermal cracking proceeds through neutral reactor species called free radicals. Another use for thermal cracking is to convert the bottom of the barrel into usable products such as fuel oils. Presently thermal cracking is not a significant process in a refinery within the United States because the gasoline that is produced from it would work properly in current automobiles. This is because the current automobiles require a fuel with a higher octane rating. This lead to the introduction of catalytic cracking.
Thermal Cracking
Thermal cracking is the chemical process of converting larger, long straight chain alkanes found in gas oils and other crude oil fractions into shorter straight chain alkanes. These shorter alkane chains are more desired because of their use in transportation fuels like gasoline. The thermal cracking reactions are governed by free radicals. The chain reaction of free radicals starts by breaking the C-C bond in the alkanes. This forms two free radicals. This step is called the initiation reaction. The next step, the propagation reaction produces a short chain alkane and one radial, which continues the chain. The final step is the termination reaction. This process started in the 1900’s as a way to increase the yield of motor gasoline from crude oils. These high-octane fuels were used in aircraft. Catalytic cracking came into use in the 1930’s and 1940’s. Because the catalytic cracking process produced higher yields of gasoline with high octane numbers, thermal cracking is no longer a method for breaking longer chains into shorter chains for gasoline production in modern refineries. In locations where diesel fuels are in high demand thermal cracking is still used. The use of thermal cracking in modern refineries is limited to naphtha cracking of residual fractions like vacuum distillation residue.
Thermal Cracking History and Modern Techniques
Thermal cracking is a process that produces short straight chain paraffin from longer straight chains found in gas oils and other heavier crude oil fractions. The chemistry of thermal cracking involves free radicals that are reactive species with unpaired electrons but have a neutral electronic charge. It is the free radical chemistry that is responsible for producing gasoline with a relatively low octane number.
A Russian engineer named Vladimir Shukov introduced the first thermal cracking method in the Russian Empire in 1891. However, it was much later in 1912 that William Merriam Burton and Robert E. Humphreys designed a similar thermal cracking process which operated under temperature conditions of 700 to 750 °F and an absolute pressure of 90 psi. The advantage of the system they developed was that both the condenser and the boiler were continuously kept under pressure. A few years later in 1921, an employee at the Universal Oil Products Company, C.P. Dubbs, developed a more advanced technique which operated at higher temperatures of 750–860 °F. The design became known as the Dubbs process and was extensively used until the early 1940s.
Modern day techniques of thermal processing include visbreaking and coking. Visbreaking is a mild fom of thermal cracking whereby the viscosity of the heavy crude oil residue is lowered significantly without affecting the boiling point range. Temperatures of about 950° F are used in the distillation column. Visbreaking mostly depends on temperature and time of the reaction. Coking is a severe form of thermal cracking that is used to convert heavy residuals into lighter more useful products and distillates. The most common coking techniques include delayed coking, fluid coking and flexi coking.
Sources:
Wikipedia: http://en.wikipedia.org/wiki/Cracking_(chemistry)
Course Webpage: https://www.e-education.psu.edu/fsc432/content/chemistry-thermal-cracking
Set Laboratories: http://www.setlaboratories.com/therm/tabid/107/Default.aspx
Significance of Thermal Cracking in Petroleum Refining
Thermal cracking has played a large role in petroleum refining for many years. The first technique of thermal cracking was invented and patented by a Russian engineer in 1891. Ever since its invention, Thermal cracking has been used in the petroleum refining industry to “crack” longer, heavier alkane chains into smaller, lighter alkane chains. This is beneficial to the refining process because it allows a larger yield of lighter products to be created from the heavier less desirable products of refining.
Free radicals, are the mechanisms that allow thermal cracking to be possible. It is because of this free radical chemistry that refineries can use the thermal cracking of gas oil to produce higher yields of low octane number gasoline.
There are three main types of reactions involved in thermal cracking. The three types are initiation, propagation and termination reactions which also occur in that order. During the first step, or the initiation reaction, a single molecule is broken into two free radicals. Then during the propagation reaction one of three types can occur, whether it be hydrogen abstraction, radical decomposition or radical addition, all propagation reactions involve the manipulation of a radical into a different radical. Finally, during the termination reaction, two free radicals are essentially terminated, forming a new, shorter, molecule than the one which was originally initiated.
Thermal cracking produces shorter straight-chain alkanes and olefins but lacks the presence of branched iso-alkanes. It is for this reason that catalytic cracking is highly favored over thermal cracking in the production of high octane gasolines.
References:
1. http://en.wikipedia.org/wiki/Cracking_(chemistry)#History_and_patents
The past and present of Thermal Cracking
Thermal cracking was first developed by William Merriam Burton in 1913 which operated under the temperature 700 F -750 F and pressure at 90 psi. It is probably the first commercialized cracking process. By this time, it is also the history of cracking of heavy crude oil fraction to light fraction started. However, as we learned from lesson 5, thermal cracking produces short straight chain alkanes from longer straight chains found in gas oils and the reactions were governed by the free radicals. The process actually produces gasoline that contain lower octane number than that of gasoline which produced by catalytic cracking. It is due to isomerization of free radicals is not favored. During 1920, the petroleum refining industry was facing a challenge called engine knock. In order to solve the problem, it needed more powerful and stranger engines, but that means it also require gasoline which contain higher octane number. By 1930, gasoline had achieved the octane ring around 60 and 70. The newer and more powerful engine required higher octane gasoline which up to about 100, but thermal cracking could not raise its octane number any higher. It is also the reason that thermal cracking is not common process in U.S refineries. Thermal cracking once was the primary process for distillate fuel production. Even it is not anymore, it still remain as an important process in refineries to produce diesel fuel and ethylene.
The Significance of Thermal Cracking in the Refining Industry
Thermal cracking is a process that manipulates long straight chains found in gas oils and other crude fractions, into shorter straight chain alkanes. The chemistry involves free radical reactions which are the key factor concerning the relatively low octane numbers of gas oils that undergo thermal cracking. Without this process, vacuum distillation residue (VDR) would essentially be a useless byproduct. Thermal cracking allows this residue to be converted into distillate fuels along with its primary goal – coke.
Thermal cracking was initially introduced in the early 1900s in order to produce more motor gasoline and high-octane gasoline for aircrafts. It wasn’t until the 1930s and 1940s, when catalytic cracking was introduced, that the petroleum industry seemed to lose its interest in thermal cracking. Today, there is still a desire for such a process, mainly in countries where the chief petroleum fuel in high demand is diesel fuel. Thermal cracking is also used for VDR with visbreaking and coking processes.
There are two (but technically three) main types of thermal cracking during coking, which include delayed coking and fluid coking. The third is known as flexi-coking, a derivative of fluid coking utilized to maximize the yield of distillate products. There is a notable market for this rejected carbon since coke has economic value as it can be used as fuel or as filler when producing anodes for the electrolysis of alumina.
Significance of Thermal Cracking
As mentioned in the lesson, the separation process of crude oil provides insufficient yields for the desired products i.e. gasoline. To satisfy the demand for more desirable products, conversion processes are used to enhance the yield. Cracking is the process of breaking down large molecules into smaller ones. Free radicals are the active intermediate species in thermal cracking (as opposed to ions in catalytic cracking). Though radicals are more stable on ternary or secondary carbons, the weakest bond in a compound is broken and the radical is typically produced on a primary carbon. Additionally, due to the fact that beta-bond scission reactions proceed faster than isomerization in a radical during the thermal cracking process, the final product is primarily composed of straight-chain parrafins and negligible amounts of branched-chain paraffins. Straight-chain parrafins have a lower octane rating – or a higher tendency to knock (self ignite) – an undesirable effect in modern gasoline engines. Therefore, presently thermal cracking is rarely used to improve gasoline yields and instead used to convert heavy gas oils into light gas oils with some byproducts of gas, gasoline, and fuel oil. However, it is still used to improve diesel yields (where knocking is desirable) in countries that primarily rely on diesel fuel.
The History of Thermal Cracking
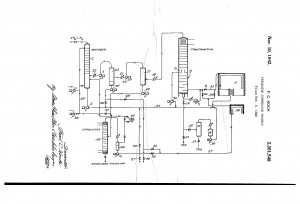 Fig. 1: “Petroleum conversion process” by Fred Koch (Source)
Fig. 1: “Petroleum conversion process” by Fred Koch (Source)
Now that we have exhausted the virtually endless range of separation processes, we concentrate on the set of thermal processes that engrained the oil and gas industry in transportation. Unlike the separation byproduct, asphalt, which is one of many pavement options, the products of thermal conversion processes are inimitable in today’s American market (unless, of course, you are an electric motor fanatic like me!). Before delving into the invaluable products, we review a brief history lesson on thermal processes in refineries.
Chemical engineer, Fred Koch, might not be the father of thermal processes, but he certainly is credited with “spreading the wealth”. Fig. 1 (above) references his patent that enabled smaller entities to profit in the spoils of big oil and gasoline production. Ironically, despite building his chemicals empire in the Soviet Union, the esteemed MIT graduate took a strong political stance against communism. But, I digress!
 Fig. 2: Fred Koch (Source)
Fig. 2: Fred Koch (Source)
The use of automobiles increased the demand for gasoline beyond the rate of straight-run gasoline distillation. Despite a sharp increase in drilling activity, the refining industry required more light gas oil through the thermal processing of the heavy gas oil and vacuum distillate residue (VDR). Conveniently, the first applications fueled WWI and subsequent wars. Thermal cracking chiefly produces in light gas oil, gasoline, residual fuel oil, and petroleum coke. While the demand for these products has only increased since its inception, catalytic cracking has usurped the attention of industry and academia alike.
Recent concerns of aging refineries call for enhanced control systems. Perhaps, the most hazardous process takes place in the coking drums, where three phase system could lead to unexpected pressure changes. Flowserve provides an example of this complex system. An intriguing mechatronic advancement involves the use of automated decoking systems. As long as thermal cracking provides ethylene production for the petrochemical industry, engineers will continue to develop best practices for safe operation of these units.
