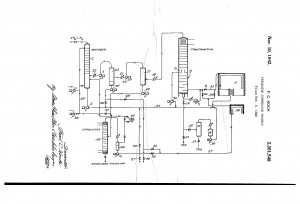
 Fig. 1: “Petroleum conversion process” by Fred Koch (Source)
Fig. 1: “Petroleum conversion process” by Fred Koch (Source)
Now that we have exhausted the virtually endless range of separation processes, we concentrate on the set of thermal processes that engrained the oil and gas industry in transportation. Unlike the separation byproduct, asphalt, which is one of many pavement options, the products of thermal conversion processes are inimitable in today’s American market (unless, of course, you are an electric motor fanatic like me!). Before delving into the invaluable products, we review a brief history lesson on thermal processes in refineries.
Chemical engineer, Fred Koch, might not be the father of thermal processes, but he certainly is credited with “spreading the wealth”. Fig. 1 (above) references his patent that enabled smaller entities to profit in the spoils of big oil and gasoline production. Ironically, despite building his chemicals empire in the Soviet Union, the esteemed MIT graduate took a strong political stance against communism. But, I digress!
 Fig. 2: Fred Koch (Source)
Fig. 2: Fred Koch (Source)
The use of automobiles increased the demand for gasoline beyond the rate of straight-run gasoline distillation. Despite a sharp increase in drilling activity, the refining industry required more light gas oil through the thermal processing of the heavy gas oil and vacuum distillate residue (VDR). Conveniently, the first applications fueled WWI and subsequent wars. Thermal cracking chiefly produces in light gas oil, gasoline, residual fuel oil, and petroleum coke. While the demand for these products has only increased since its inception, catalytic cracking has usurped the attention of industry and academia alike.
Recent concerns of aging refineries call for enhanced control systems. Perhaps, the most hazardous process takes place in the coking drums, where three phase system could lead to unexpected pressure changes. Flowserve provides an example of this complex system. An intriguing mechatronic advancement involves the use of automated decoking systems. As long as thermal cracking provides ethylene production for the petrochemical industry, engineers will continue to develop best practices for safe operation of these units.